Blood Pressure Medications Recalled By The Fda
Click here to read the full article. While many of the food recalls we cover are made out of an abundance of caution recalls involving medications are naturally a.
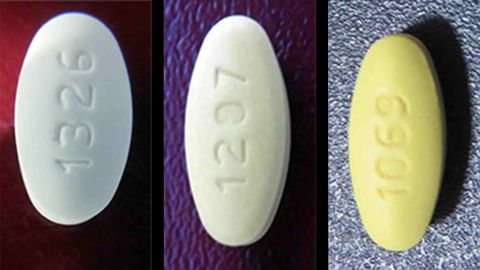
Fda Warns Of Common Blood Pressure Medicine Shortage Due To Recalls Cnn
Blood pressure medication made by Lupin Pharmaceuticals Inc.

Blood pressure medications recalled by the fda. MONTREAL -- A trio of high blood pressure medications are being recalled due to the presence of an impurity. Continue Reading Show full articles without Continue Reading. The likelihood of it causing cancer is extremely small according to the FDA.
The recalled blood pressure medication has an impurity above the specification limit that. The FDA said in a March 22 posting on its website that it has updated the list of valsartan medicines under recall to incorporate additional repackagers of Aurobindos valsartan-containing medicine. Stop taking these pills now.
High Blood Pressure Medicine Recalled by the FDA September 23 2019 By Amanda Blankenship Leave a Comment It isnt the first time the. Food Drug Administration FDA recalls products weekly that can be found on their siteIt is not only high blood pressure medication that they recall. Several companies are recalling Irbesartan Iosartan and valsartan due to the presence.
Roston - Oct 15 2021 744pm CDT. A blood pressure medication is being recalled for possibly containing high levels of a cancer-causing impurity according to a recall notice. Blood pressure pill recall.
Irbesartan tablets as well as its Irbesartan and Hydrochlorothiazide tablets. This includes some combination tablets which contain valsartan and amlodipine or valsartan amlodipine and hydrochlorothiazide. The FDA has published a new recall advisory from Lupin Pharmaceuticals over.
FDA has issued a recall of certain lots of angiotensin II receptor blocker ARB high blood pressure medication containing valsartan losartan or irbesartan. Blood Pressure Medications Recalled for Possible High Carcinogen Levels. FDA blood pressure medicine recall.
In regards to the recent blood pressure medication recalls by the FDA that started back in July of 2018As the announcement states it is a recall of several drug products containing the active ingredient. Two blood pressure medications are being recalled by Lupin Pharmeceuticals the FDA said. Lupin has discontinued marketing both medications as of Jan.
A representational image of. However if youre taking one particular type of blood pressure medication your health could be at risk now that its subject. Is being recalled by the US.
The post FDA blood pressure medicine recall. 18 2021 -- Two types of blood pressure medication made by Lupin Pharmaceuticals have been recalled due to potential. Even if your blood pressure medication is on the recall list there is no need to panic.
Stop taking these pills now appeared first on BGR. 115 likes 187 shares. April 5 2019 -- The FDA on Thursday issued a list of 40 blood pressure medicines it found free of contamination with the chemical nitrosamine an.
What to do if you have these medicines in your cabinet or store Lupin Pharmaceuticals is voluntarily recalling two types of. 11 rows FDA provides a searchable list of recalled products. Estimates indicate that if 8000 people took the highest dose of one of the contaminated prescriptions for four years it would result in one additional case of cancer.
FDA Issues List of Safe Blood Pressure Meds. Blood pressure and fluid retention drugs recalled over cancer concerns. Drug recalls are actions taken by.
Why are these medications being recalled. If you have high blood pressure youre far from aloneAccording to the Centers for Disease Control and Prevention CDC approximately 45 percent of Americans have been diagnosed with hypertension or take medication for the condition. This story on the recall of certain blood pressure medications has been updated numerous times since it was first published on July 24 2018.
Food and Drug Administration FDA for possibly containing a cancer-causing impurity. FDA is alerting the public about a voluntary recall of several drug products containing valsartan used to treat high blood pressure and heart failure.
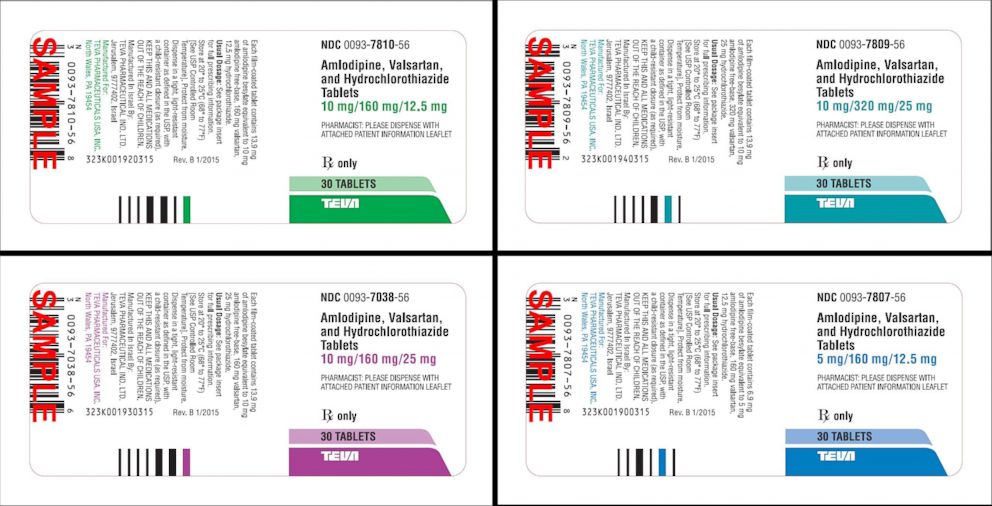
Blood Pressure Medication Recall What You Need To Know Abc News

Losartan Blood Pressure Medication Recall Expanded Again Over Cancer Concerns Fda Says Fox News
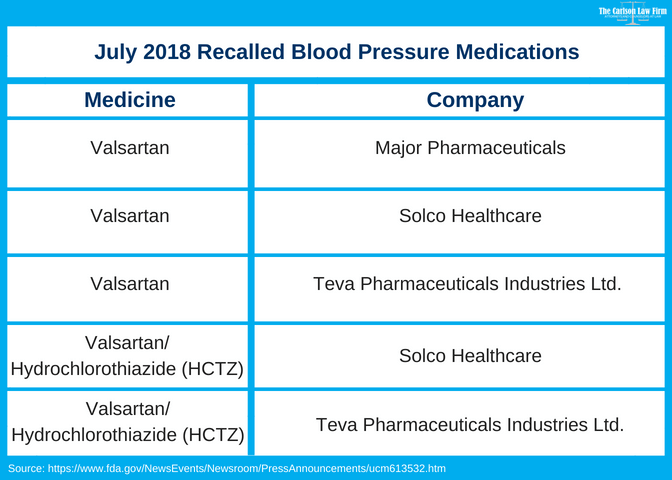
Valsartan Recall Fda Finds Carcinogenic Impurity In Blood Pressure Meds
Nationwide Recall Of Certain High Blood Pressure And Heart Failure Medications Issued Wztv

Blood Pressure Medication Recall Expanded By Fda Kutv
Fda Recalls Blood Pressure Medication Containing Valsartan Norton Healthcare Louisville Ky
Blood Pressure Medication Recall Drugs Recalled Due To Cancer Risk Al Com
Blood Pressure Medication Recall By The Fda Learn More Physiostasis
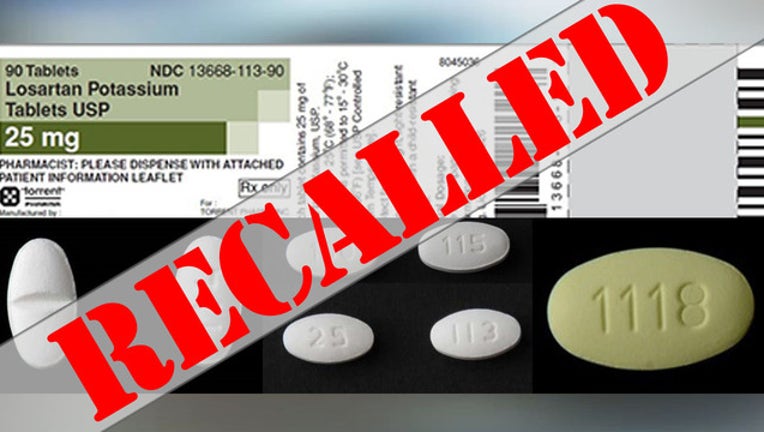
Blood Pressure Medication Recall Expanded After Possible Cancer Causing Impurity Detected Says Fda
/cloudfront-us-east-1.images.arcpublishing.com/gray/HJZ5B42YZNEJNDWE6MAMLCX2FA.jpg)
Blood Pressure Medication Recalled Due To Unexpected Impurity

Blood Pressure Medication Recalled By Fda Hsa Cease Distribution Loop Cayman Islands
Hydrochlorothiazide Is Being Recalled For A Labeling Mistake Time

Fda Recalls Popular Blood Pressure Med Losartan For Possible Cancer Causing Impurity Manchester Ink Link
Valsartan Losartan And Other Blood Pressure Medication Recalls 2018 19

Fda Expands Blood Pressure And Heart Medication Recall Expanded
Fda Recalls Blood Pressure Medicine After Finding Carcinogen In Pills Phillyvoice




Comments
Post a Comment