Blood Pressure Medications Recalled
This includes some combination tablets which contain valsartan and amlodipine or valsartan amlodipine and hydrochlorothiazide. Patients who are taking the recalled medication are advised to continue taking the Irbesartan.
A Lupin Pharmaceuticals Inc.

Blood pressure medications recalled. October 19 2021 704 AM 2 min read. Losartan is a medication used to treat high blood pressure. Blood pressure medication is being recalled by the US.
Doctors regularly prescribed antihypertensive drugs like Losartan Valsartan and Irbesartan to millions of patients in the United States and worldwide. All of the affected medicines contain irbesartan which is used. Why Colin Powells death after COVID-19 vaccination is rare Colorado Springs Colorado.
Is voluntarily recalling its Irbesartan and Hydrochlorothiazide tablets at the consumer level. Blood Pressure Medications Recalled for Possible High Carcinogen Levels. The affected products all contained valsartan losartan.
A blood pressure medication is being recalled for possibly containing high levels of a cancer-causing impurity according to a recall notice from the US. 18 2021 Two types of blood pressure medication made by Lupin Pharmaceuticals have been recalled due to potential high levels of a cancer-causing substance according to an. Blood pressure medication made by Lupin Pharmaceuticals Inc.
Dozens of medications used to treat high. As Covid cases drop in Georgia and Florida some states with colder weather see an increase Atlanta Georgia. Food and Drug Administration for possibly containing a cancer-causing impurityThe voluntary recall.
A blood pressure medication is being recalled for possibly containing high levels of a cancer-causing impurity according to a recall notice. Blood pressure medication recalled over risk of cancer-causing impurity. Food and Drug Administration FDA.
A Lupin Pharmaceuticals Inc. The expiration date of the medication is March 2022. Blood Pressure Medication Recall 2021.
Blood Pressure Medications Recalled for Possible High Carcinogen Levels. Dozens of blood pressure medications have been recalled since the first products were pulled off the shelf in July 2018 due to impurities. 18 2021 -- Two types of blood pressure medication made by Lupin Pharmaceuticals have been recalled due to potential.
Another 25 batches of high blood pressure pills have been recalled over fears they may cause cancer health chiefs announced today. Blood pressure pills recalled over cancer risk. In another recall over cancer-causing impurity levels Lupin Pharmaceutical has recalled several batches of its Irbesartan tablets and Irbesartan and Hydrochlorothiazide tablets because N-nitrosoirbesartan a substance that causes cancer - was found in.
1170 rows Find out which specific blood pressure medications are affected by the recall. Hypertension Drug Recalled Over Cancer Risks. Food and Drug Administration FDA for potentially containing a.
Leave a Comment Lawsuits By Amy Gilmore. Food and Drug Administration FDA for potentially containing a probable human carcinogen The voluntary recall includes the companys Irbesartan tablets and Hydrochlorothiazide tablets at the consumer level. Blood pressure medication recalled over risk of cancer-causing impurity Cleveland Ohio.
A trio of high blood pressure medications are being recalled due to the presence of an impurity. More than 30 batches of pills used to treat high blood pressure have been contaminated by an impurity that can increase the risk of. FDA has issued a recall of certain lots of angiotensin II receptor blocker ARB high blood pressure medication containing valsartan losartan or irbesartan.
The recalled Telmisartan tablets have a lot number of 1905005661 and an NDC number of 62332-087-30. In 2018 and 2019 some batches of losartan medications were voluntarily recalled because they were found to contain compounds that were not listed in the ingredients. No adverse events have been reported by people taking these medications.
23 2019 with an additional losartan recall from Torrent Pharmaceuticals. The Food and Drug Administration has recently recalled a number of blood pressure medications after discovering that they contained potential cancer-causing contaminants. This story was updated on Sept.
Is being recalled by the US. Is recalling its Irbesartan and Hydrochlorothiazide tablets after an analysis revealed that certain API batches contained the impurity N-nitrosoirbesartan at higher-than-allowed levels. Losartan Recall Lawsuit 2021 Blood Pressure Drug Recalled.
Patients prescribed angiotensin II receptor medications have filed Losartan recall lawsuits against drug manufacturers. Facebook Twitter LinkedIn Tumblr Pinterest Reddit VKontakte Pocket Skype WhatsApp Telegram Viber. Blood pressure medication is being recalled by the US.
A full list of Irbesartan and Hydrochlorothiazide tablets recalled can be viewed here.

Fda More High Blood Pressure Medications Recalled Due To Cancer Risk Wsyx
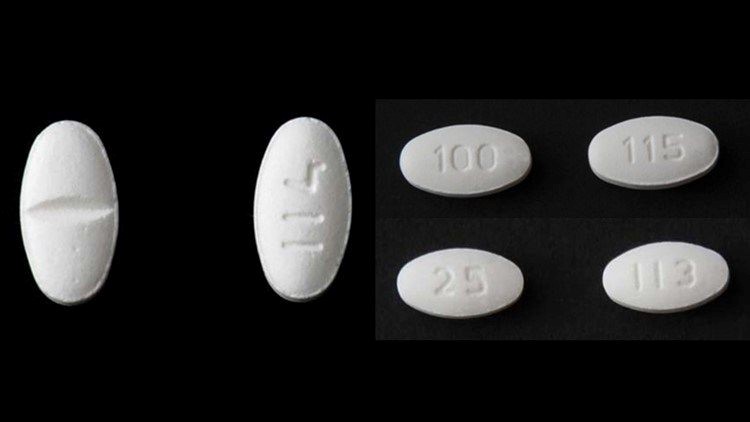
More Blood Pressure Medications Recalled For Cancer Causing Impurity Cbs8 Com

Cancer Causing Chemicals Spark Recall Of Blood Pressure Drugs Over Contamination Fears

More Blood Pressure Medication Recalled After Cancer Causing Chemical Found

Losartan Blood Pressure Medication Recall Expanded Again Over Cancer Concerns Fda Says Fox News
Blood Pressure Medication Recall Expands Again To Include Losartan Wlos
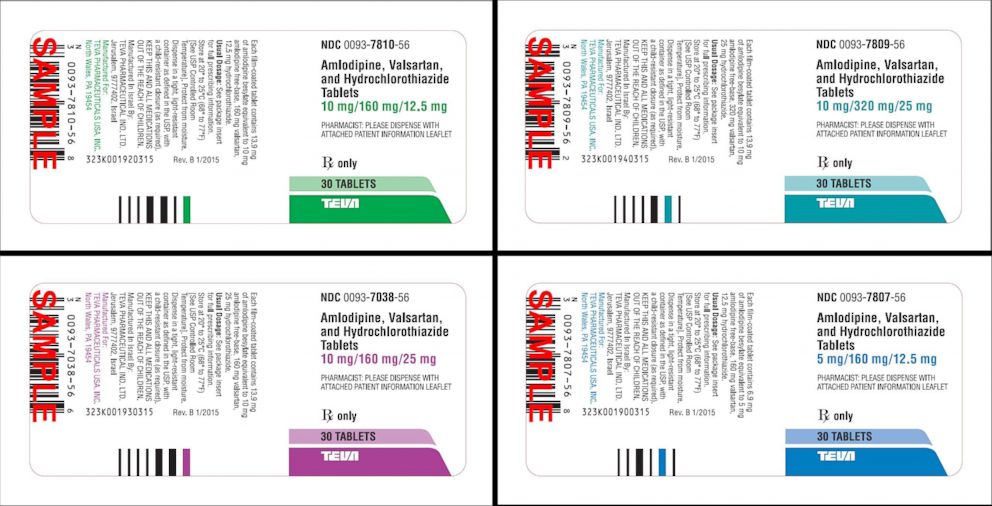
Blood Pressure Medication Recall What You Need To Know Abc News

Blood Pressure Medication Recall What You Need To Know Abc News

Blood Pressure Medication Recalled Because May Contain Stronger Dose Than Indicated Pennlive Com

Blood Pressure Medication Recalled For Label Mix Up Silive Com
/cloudfront-us-east-1.images.arcpublishing.com/gray/IFRWTBMGXFAAVL36BTAQD77TQ4.png)

/cloudfront-us-east-1.images.arcpublishing.com/gray/A7A5CIT5GREO7OVZPSG4ZHG2U4.png)

/cloudfront-us-east-1.images.arcpublishing.com/gray/MYC4NDVDSNFBPDGTVLYJEP43JA.png)


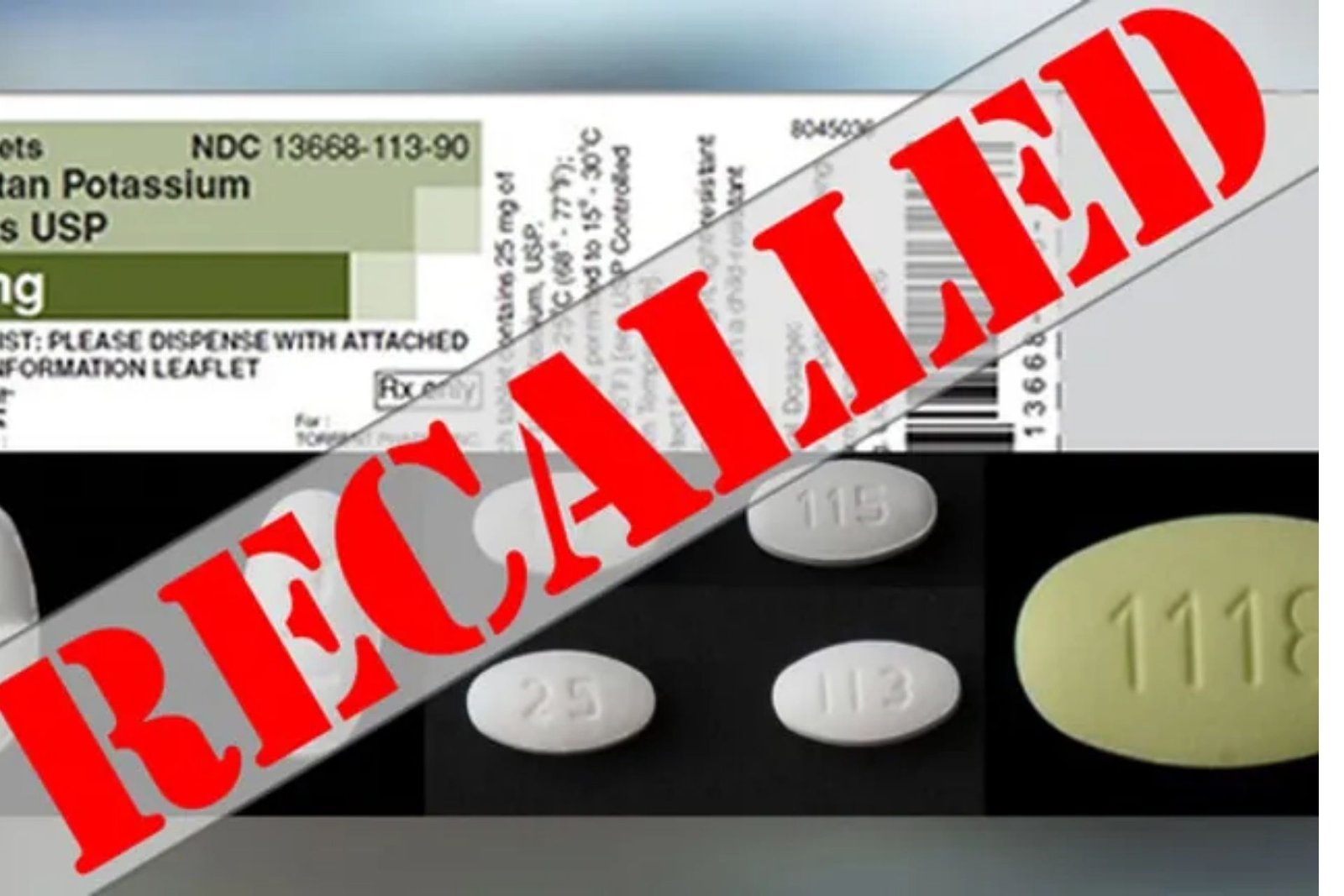



Comments
Post a Comment