Blood Pressure Meds Recalled
FDA has issued a recall of certain lots of angiotensin II receptor blocker ARB high blood pressure medication containing valsartan losartan or irbesartan. September 24 2019 952 P.

Blood Pressure Medication Recalled After Trace Amounts Of Cancer Causing Chemical Found Cbs News
Blood Pressure Medication Recall Continues Heres What You Need to Know Written by Shawn Radcliffe Updated on September 27 2019 Several high blood pressure drugs were recalled due to.

Blood pressure meds recalled. What you need to know. Doctors use the medication to treat hypertension low blood pressure and symptoms in patients with type 2 diabetes. A Lupin Pharmaceuticals Inc.
Hypertension Drug Recalled Over Cancer Risks. Blood pressure medication recalled over risk of cancer-causing impurity Cleveland Ohio. Blood pressure medication recalled over risk of cancer-causing impurity NEXSTAR Lupin Pharmaceuticals Inc.
Blood Pressure Medication Recall 2021. A Lupin Pharmaceuticals Inc. Roston - Oct 15 2021.
This story was updated on Sept. Blood pressure medication recalled over risk of cancer-causing impurity News. Food and Drug Administration FDA for potentially containing a probable human carcinogen.
In another recall over cancer-causing impurity levels. By Dawn Geske 101421 AT 152 PM. Blood pressure pill recall.
Why are these medications being recalled. Is voluntarily recalling a blood pressure medication that possibly contains high levels of a cancer-causing impurity according to the. A recall has been issued by.
Blood pressure and fluid retention drugs recalled over cancer concerns. Blood pressure medication is being recalled by the US. COMMON blood pressure drugs have been recalled over contamination fears with a substance that can increase the risk of cancer.
Hypertension Drug Recalled Over Cancer Risks. Published Saturday October 2. Blood pressure medication recall.
By Dawn Geske 032521 AT 1015 AM. The UK medicine regulator today issued a recall for 31 batches of prod. Jocelina Joiner Nexstar Media Wire.
STATEN ISLAND NY. Stop taking these pills now. The Food and Drug.
November 1 2018 -- Another high blood pressure drug is being recalled due to contamination that. FDA blood pressure medicine recall. Dozens of medications used to treat high blood pressure have been recalled over the.
Roston - Oct 15 2021 744pm CDT. Food and Drug Administration FDA for potentially containing a probable human carcinogen The voluntary recall includes the companys Irbesartan tablets and Hydrochlorothiazide tablets at the consumer level. 23 2019 with an additional losartan recall from Torrent Pharmaceuticals.
Blood pressure medication is being recalled by the US. Food and Drug Administration. Blood Pressure Medication Recall 2021.
Hypertension Drug Recalled Over Potential Death Risk. Second Blood Pressure Med Recalled for Contamination. The FDA continues to update the list of medications being recalled.
Oct 19 2021 0234 PM. October 19 2021 704 AM 2 min read. Blood Pressure Medication Recall 2021.
What to do if you have these medicines in your cabinet or store News Instacart is acquiring a maker of smart grocery carts wading deeper into physical retail. As such consumers who took these drugs may want to discuss their cancer risk with a physician. All of the affected medicines contain irbesartan which is.
A blood pressure medication is being recalled for possibly containing high levels of a cancer-causing impurity according to. Rowe CTVNewsMontrealca Digital Reporter DanielJRowe42 Contact. The recall affects 31 batches of drugs containing Irbesartan and 2 containing Losartan medicines which are used to treat high blood pressure heart failure type 2.
Blood pressure medication recalled due to an impurity at unacceptable levels. This includes some combination tablets which contain valsartan and amlodipine or valsartan amlodipine and hydrochlorothiazide. Two types of blood pressure medications sold by Lupin Pharmaceuticals Inc.
Another 25 batches of high blood pressure pills have been recalled over fears they may cause cancer health chiefs announced today. The FDA has published a new. In another recall over cancer-causing impurity levels Lupin Pharmaceutical has recalled several batches of its Irbesartan tablets and Irbesartan and Hydrochlorothiazide tablets because N-nitrosoirbesartan a substance that causes cancer - was found in.

Blood Pressure Medication Recalled For Label Mix Up Silive Com

High Blood Pressure Medication Recalled Wild Coast Compass

Blood Pressure Drug Recalled Over Risky Label Mix Up Everyday Health

Cancer Causing Chemicals Spark Recall Of Blood Pressure Drugs Over Contamination Fears

Recall Expanded For Blood Pressure Medication Due To Potential Cancer Risk Abc7 Los Angeles
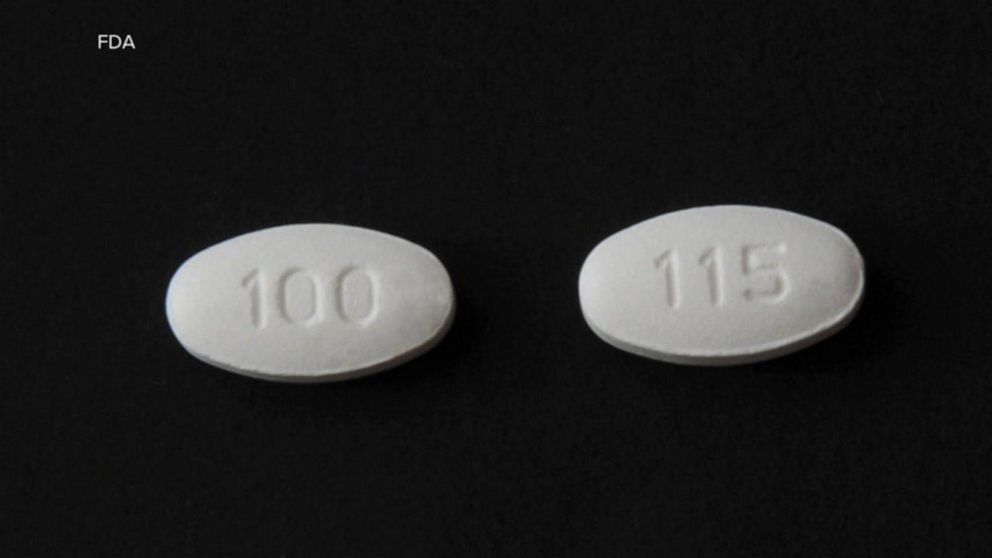
Blood Pressure Medication Recall What You Need To Know Abc News

Blood Pressure Drug Recalled After Probable Cancer Causing Ingredient Detected Fox News

Fda Recalls More High Blood Pressure Medicine Due To Potential Cancer Risk Fox 59

Common Blood Pressure Drug Recalled Due To Cancer Causing Chemicals Wales Online

Blood Pressure Medication Recalled Because May Contain Stronger Dose Than Indicated Pennlive Com

More Blood Pressure Medication Recalled After Cancer Causing Chemical Found

Blood Pressure Medication Recalled Youtube
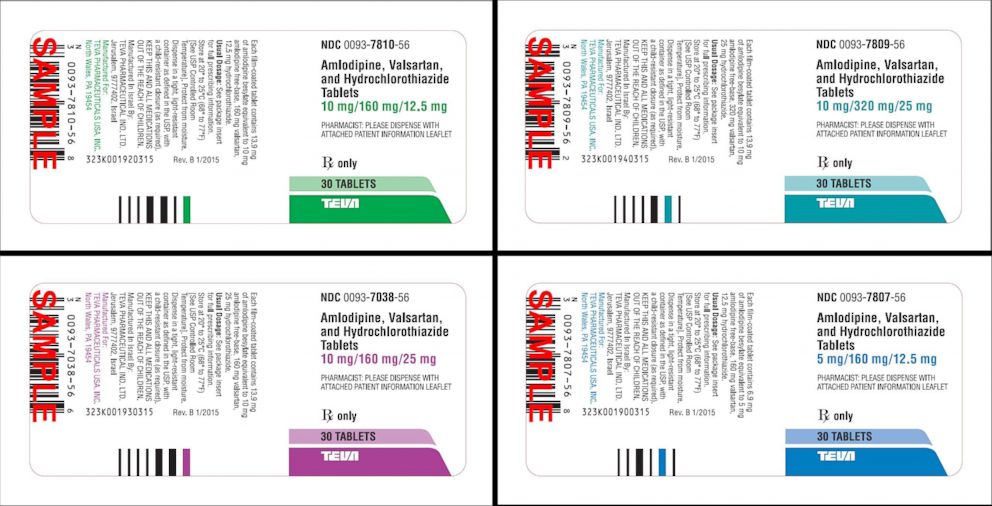
Blood Pressure Medication Recall What You Need To Know Abc News

Blood Pressure Meds Recalled Over Contamination Concern Wfmj Com



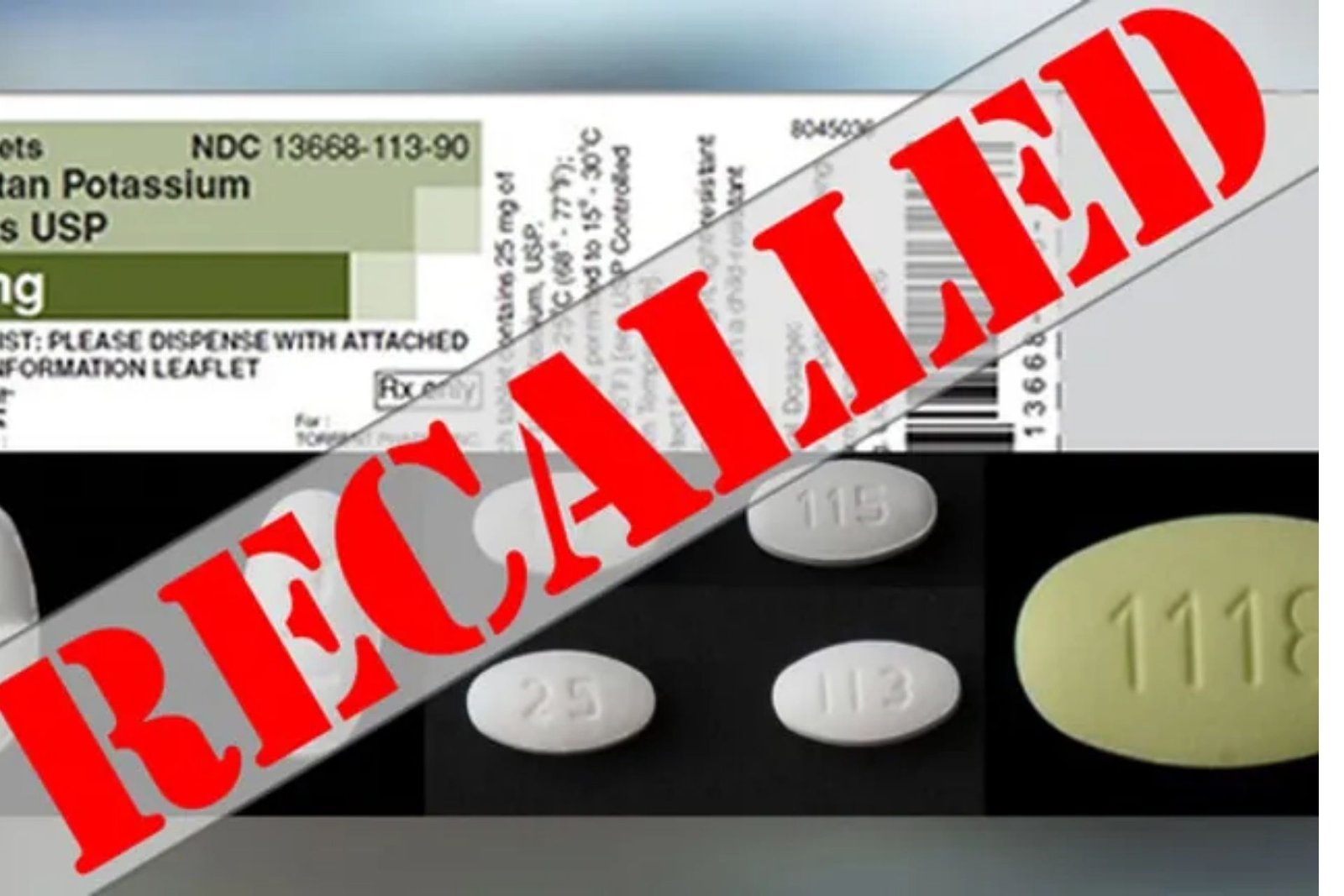
Comments
Post a Comment